What Does The K Value Mean In Chemistry . a large value of the equilibrium constant \(k\) means that products predominate at equilibrium; knowing the value of the equilibrium constant, k k, will allow us to determine: When the k value is high for a. the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. the equilibrium constant may be defined as the ratio between the product of the molar concentrations of the products to that of. (1) he direction a reaction will. It uses the concentrations and. When the k value is low for a reaction, this means we have more reactants than products. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. what does it represent?
from www.slideserve.com
It uses the concentrations and. what does it represent? The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. (1) he direction a reaction will. knowing the value of the equilibrium constant, k k, will allow us to determine: When the k value is high for a. a large value of the equilibrium constant \(k\) means that products predominate at equilibrium; the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: the equilibrium constant may be defined as the ratio between the product of the molar concentrations of the products to that of.
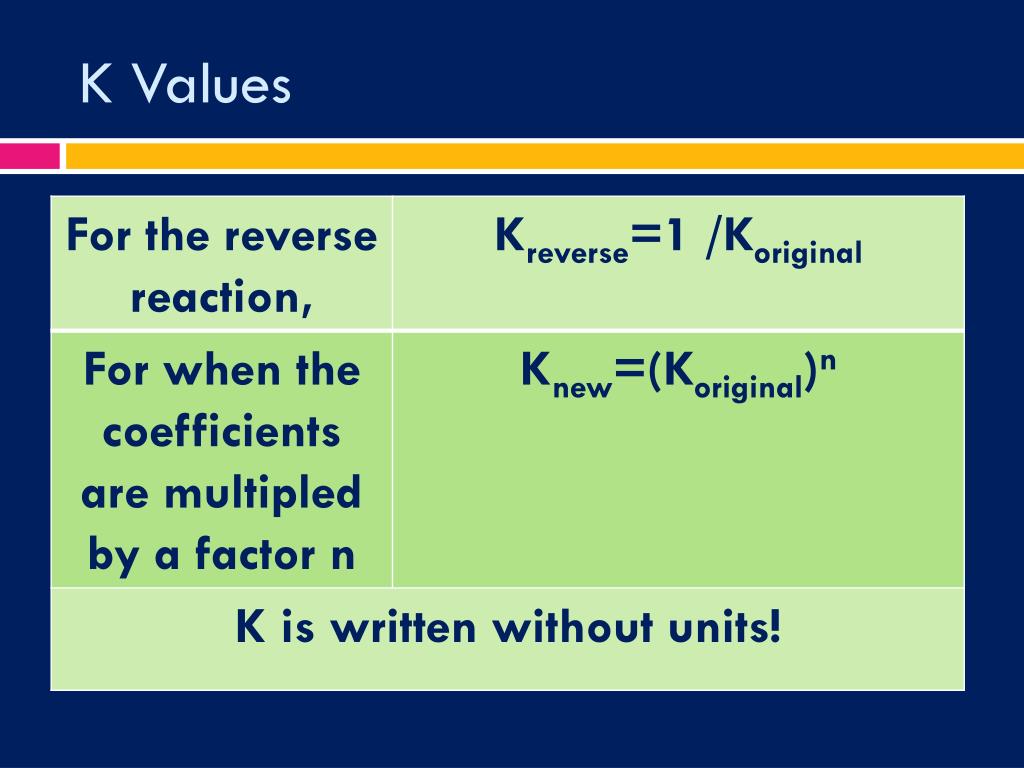
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
What Does The K Value Mean In Chemistry When the k value is high for a. It uses the concentrations and. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. the equilibrium constant may be defined as the ratio between the product of the molar concentrations of the products to that of. knowing the value of the equilibrium constant, k k, will allow us to determine: When the k value is high for a. a large value of the equilibrium constant \(k\) means that products predominate at equilibrium; (1) he direction a reaction will. When the k value is low for a reaction, this means we have more reactants than products. the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: what does it represent? the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Does The K Value Mean In Chemistry When the k value is high for a. When the k value is low for a reaction, this means we have more reactants than products. a large value of the equilibrium constant \(k\) means that products predominate at equilibrium; the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of. What Does The K Value Mean In Chemistry.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps What Does The K Value Mean In Chemistry the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. a large value of the equilibrium constant \(k\) means that products predominate at equilibrium; knowing the value of the equilibrium constant, k k, will allow us to determine: When the k value is high. What Does The K Value Mean In Chemistry.
From worksheetaidansuskm.z13.web.core.windows.net
Chemical Equilibrium Formula Sheet What Does The K Value Mean In Chemistry When the k value is low for a reaction, this means we have more reactants than products. what does it represent? It uses the concentrations and. the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. . What Does The K Value Mean In Chemistry.
From wiki.whitson.com
Equilibrium Ratios whitson wiki What Does The K Value Mean In Chemistry the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: When the k value is low for a reaction, this means we have more reactants than products. (1) he direction a reaction will. the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of. What Does The K Value Mean In Chemistry.
From saylordotorg.github.io
The Equilibrium Constant What Does The K Value Mean In Chemistry When the k value is high for a. (1) he direction a reaction will. the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. It uses the concentrations and. knowing the value of the equilibrium constant, k k, will allow us to determine: what. What Does The K Value Mean In Chemistry.
From chem.libretexts.org
Gas Equilibrium Constants Kc And Kp Chemistry LibreTexts What Does The K Value Mean In Chemistry the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. When the k value is low for a reaction, this means we have more reactants than products. (1) he direction a reaction will. the magnitude of the equilibrium constant, k, indicates the extent to which. What Does The K Value Mean In Chemistry.
From www.youtube.com
CHEM 201 Calculating Equilibrium Concentrations from K and Initial What Does The K Value Mean In Chemistry (1) he direction a reaction will. When the k value is low for a reaction, this means we have more reactants than products. the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. a large value of the equilibrium constant \(k\) means that products predominate. What Does The K Value Mean In Chemistry.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID What Does The K Value Mean In Chemistry (1) he direction a reaction will. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. what does it represent? the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. the equilibrium constant may be defined as the ratio. What Does The K Value Mean In Chemistry.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Unit 11, Part II PowerPoint Presentation What Does The K Value Mean In Chemistry the equilibrium constant may be defined as the ratio between the product of the molar concentrations of the products to that of. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. When the k value is low for a reaction, this means we have more reactants than products. knowing the value of. What Does The K Value Mean In Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Does The K Value Mean In Chemistry the equilibrium constant may be defined as the ratio between the product of the molar concentrations of the products to that of. When the k value is high for a. It uses the concentrations and. what does it represent? the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio. What Does The K Value Mean In Chemistry.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical What Does The K Value Mean In Chemistry what does it represent? When the k value is low for a reaction, this means we have more reactants than products. It uses the concentrations and. knowing the value of the equilibrium constant, k k, will allow us to determine: When the k value is high for a. the magnitude of the equilibrium constant, k, indicates the. What Does The K Value Mean In Chemistry.
From www.slideserve.com
PPT The pK a Scale PowerPoint Presentation, free download ID6598843 What Does The K Value Mean In Chemistry the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: a large value of the equilibrium constant \(k\) means that products predominate at equilibrium; the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. It uses the concentrations. What Does The K Value Mean In Chemistry.
From www.youtube.com
Relationship between Ka and Kb Chemistry Khan Academy YouTube What Does The K Value Mean In Chemistry When the k value is high for a. (1) he direction a reaction will. what does it represent? the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: It uses the concentrations and. the equilibrium constant may be defined as the ratio between the product of the molar concentrations of the. What Does The K Value Mean In Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium CHAPTER 15 Chemistry The Molecular Nature What Does The K Value Mean In Chemistry the equilibrium constant may be defined as the ratio between the product of the molar concentrations of the products to that of. a large value of the equilibrium constant \(k\) means that products predominate at equilibrium; the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations. What Does The K Value Mean In Chemistry.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and What Does The K Value Mean In Chemistry When the k value is low for a reaction, this means we have more reactants than products. the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. what does it represent? a large value of the equilibrium constant \(k\) means that products predominate at. What Does The K Value Mean In Chemistry.
From telgurus.co.uk
Explain the Lock and key mechanism in relation to enzymes. Science What Does The K Value Mean In Chemistry It uses the concentrations and. the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: the equilibrium constant may be defined as the ratio between the product of. What Does The K Value Mean In Chemistry.
From www.slideshare.net
AP Chemistry Chapter 15 Outline What Does The K Value Mean In Chemistry When the k value is low for a reaction, this means we have more reactants than products. the equilibrium constant, denoted as k eq or just k, is a numerical value that expresses the ratio of the concentrations of products. what does it represent? When the k value is high for a. the magnitude of the equilibrium. What Does The K Value Mean In Chemistry.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free What Does The K Value Mean In Chemistry what does it represent? (1) he direction a reaction will. knowing the value of the equilibrium constant, k k, will allow us to determine: the magnitude of the equilibrium constant, k, indicates the extent to which a reaction will proceed: the equilibrium constant may be defined as the ratio between the product of the molar concentrations. What Does The K Value Mean In Chemistry.